- General process of stress corrosion cracking
Stress corrosion cracking has a very obvious selectivity to the environment. All kinds of materials can only undergo stress corrosion cracking in special environments with strong stress corrosion sensitivity. Each special combination of different groups corresponds to less consistent stress. Corrosion mechanism. However, after long-term research and practice, people have come up with a common process of stress corrosion. The three-stage theory of stress corrosion cracking process summarizes the cracking rules in electrochemical processes and non-electrolytes, non-metals, and generally shows that the substances in the environment promote cracking in three stages. It also acts to relieve corrosion and can describe the general process of stress corrosion cracking.
The three stages are:1. Forming a protective film on the metal surfaceAlmost all alloys that undergo corrosion cracking can form a surface protective film or a passivation film so that the metal is not directly exposed to the corrosive medium.2. Protect the local rupture of the pancreas and form a source of corrosion or crackIf the protective film is partially damaged due to some reason, the base metal is directly in contact with the medium, the exposed metal surface forms an anode, and the surface of the unbroken protective film forms a cathode, forming a corroded battery, causing the anode metal to be corroded. At the same time, the exposed fresh surface will automatically form a film to achieve self-repair. When the destruction speed of the protective film is greater than the repair speed, the stress corrosion crack is expanded. The factors causing local damage to the surface film in the metal are:(1) Environmental factors, there are active ions in the environment that can destroy the passivation film, such as Cl-, Br-, and the like.(2) Metallurgical factors, defects on the metal surface, such as the screw dislocation of the outcrop will cause the film to break, non-metallic inclusions, grain boundaries and phase boundaries are prone to local corrosion. The size of the local defect ranges from a few atoms to a few millimeters in diameter.(3) Mechanical factors, the sliding step of the protruding surface generated under stress may cause the protective film to be torn.3. The crack expands in depth. If these two conditions are met, the corrosion pattern will be limited to pitting and crevice corrosion, so that the corrosion develops only along a narrow channel to the inside of the alloy without expanding on the surface. The expansion mechanism is as follows:(1) The tensile stress causes the film to continually rupture intermittently. When the membrane repassivation ability is in a narrow, suitable range, the corrosion proceeds along a narrow fracture, and the membrane portion is protected by the cathode.(2) At the crack or crack tip, a unique occlusion zone is formed, the crack tip metal dissolves, the anion migrates, and the pH decreases, causing the corrosion to accelerate, and the cathode reflects the generated oxygen partially diffusing into the crack tip metal, causing embrittlement. .(3) There is an active channel inside the alloy, which may be pre-existing or formed due to phase transformation, yielding, etc. during stress corrosion. Corrosion and brittle fracture advance along the active channel under tensile stress.The above process of stress corrosion cracking is shown in Fig. 1. Figure 2 illustrates the initiation and subcritical expansion of stress corrosion cracking and the rate of mechanically unstable fracture. In the figure, the A curve indicates a metal material having good plasticity; the B curve indicates a metal material having poor plasticity; and the c curve indicates a brittle material such as a H-11 mold steel after quenching.
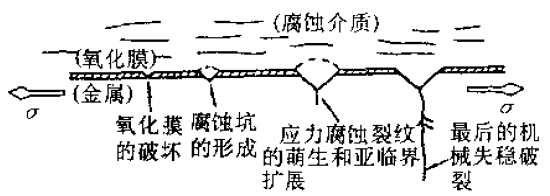 Figure 1 Schematic diagram of stress corrosion cracking process
Figure 1 Schematic diagram of stress corrosion cracking process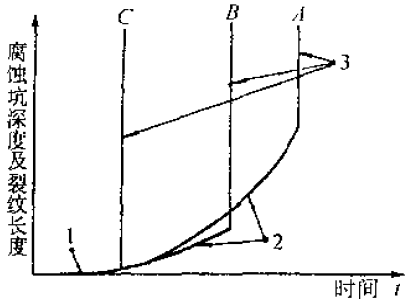 Figure 2 Schematic diagram of three stages of stress corrosion cracking
Figure 2 Schematic diagram of three stages of stress corrosion cracking1- Stage of corrosion pit generation; 2-Initiation of stress corrosion cracking and subcritical expansion stage; 3-Fast mechanical instability failure stage
Stress corrosion cracking mechanismFrom the perspective of metal physics and metal chemistry, fracture mechanics, many theories are proposed for some special stress corrosion phenomena. Each theory can explain certain phenomena and laws, but there is no theory that can explain various stress corrosion cracking laws. Therefore, the theory about the stress corrosion cracking mechanism can only be a system. It should be said that this system is still very imperfect, and a lot of research work is needed. Here, only the more popular independent theories are briefly introduced.Surface protection film rupture theoryThe basic argument of this theory is that there are different degrees of protective film, or passivation film, on the surface of the metal. If the protective film is partially damaged, a 5 day pole with the film as the cathode and the exposed metal as the anode will be formed. During the dissolution process, corrosion pits are generated, which in turn initiate stress corrosion cracking.The local damage of the surface protective film may be caused by mechanical damage, or may be caused by the sliding step caused by the sliding movement of the metal during plastic deformation, as shown in FIG. The slip step may be caused by the movement of the spiral dislocation or the edge dislocation.The size of the grain has an influence on the size of the sliding step. As shown in Fig. 4, the larger the grain, the larger the step of sliding, and the more easily the stress corrosion cracking occurs.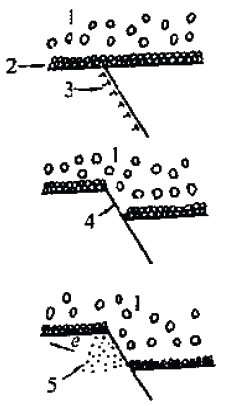 Figure 3 Local anodic dissolution process due to destruction of the surface protective film1-liquid medium; 2-protective layer; 3-metal active slip surface;4-Active surface exposed to the medium: 5-active surface is rapidly dissolvedThe size of the grain has an influence on the size of the sliding step. As shown in Fig. 4, the larger the grain, the larger the step of sliding, and the more easily the stress corrosion cracking occurs.
Figure 3 Local anodic dissolution process due to destruction of the surface protective film1-liquid medium; 2-protective layer; 3-metal active slip surface;4-Active surface exposed to the medium: 5-active surface is rapidly dissolvedThe size of the grain has an influence on the size of the sliding step. As shown in Fig. 4, the larger the grain, the larger the step of sliding, and the more easily the stress corrosion cracking occurs.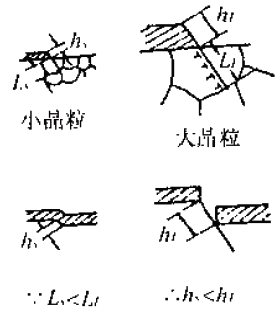 Figure 4 Schematic diagram of the effect of grain size on the slip step
Figure 4 Schematic diagram of the effect of grain size on the slip stepThe metal surface slip step may be re-passivated, and local anodic corrosion is only possible when the passivation material required for the re-passivated partial surface has been exhausted. In general, the metal surface is only re-passivated in the medium containing oxygen or oxidant. If the rate of re-passivation is greater than the rate of local anode dissolution, the stress corrosion will be prevented.
2. Active channel theoryThe basic argument of this theory is that in a metal or alloy, there is a corrosion-prone, roughly continuous path, the so-called 'active channel', along which electrochemical corrosion occurs, resulting in stress corrosion cracking. .The main factors contributing to the active channel are:a. differences in alloy composition and microstructure, such as composition segregation, multiphase alloys and grain boundary precipitates;b. highly disordered grain boundaries or subgrain boundaries where solute atoms may precipitate;c. the anode grain boundary region due to local stress concentration and resulting strain;d. rupture of the surface protective film due to strain;e. The anode region and the like due to plastic deformation.3. Anode rapid dissolution theoryThe basic argument of this theory is that stress corrosion cracking is the result of rapid continuous dissolution of the crack tip anode. The presence of stress accelerates the rate at which the anode dissolves and promotes the separation of the metal.In general, the amount of metal dissolved at the crack tip is very small, so there is no problem of concentration polarization and passivation, and the rapid dissolution of the crack tip is ensured. Tests have shown that the crack tip is much faster than the two sides of the crack, about 10^4 times.4. Stress adsorption fracture theoryThe basic argument of this theory is that the stress corrosion cracking is due to the adsorption of some special ions at the crack tip, such as C1-, OH-, etc., which weakens the affinity of the strained metal atom bond, that is, the metal surface energy is reduced. Under the action of stress, the cracking of the metal is promoted.Figure 5 shows that under the tensile stress σ, the atomic bond X of the crack tip will be in the tension state, and the shear stress will be generated on the slip surface inside the material. If a certain ion S is adsorbed on the crack tip atom at this time, the atomic bond X intensity is lowered to cause X to rupture, and the adsorption may cause the shear stress of the slip surface P to rise. If the ratio of rupture stress to shear stress decreases, the rupture mode may change from ductile to brittle.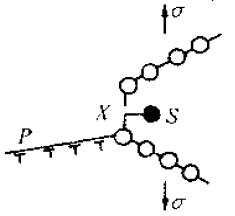 Figure 5 Schematic diagram of stress adsorption crackingAccording to the stress adsorption theory, we can know:a. For different metal materials, the adsorbed ions are selective. Some ions in some electrolytes are liable to cause stress corrosion cracking of some metal materials. For example, Cl- and HO- are suitable for austenitic stainless steel, N03- or OH- to low carbon steel, etc., which are susceptible to stress corrosion cracking;b. According to the above characteristics, it is possible to replace and dilute the harmful anions with a sufficient concentration of harmless anions. For example, by adding N03- or ions to a solution containing oxygen ions, it can prevent stress corrosion cracking of stainless steel;c. Surface stress adsorption at the crack tip is not only related to the chemical composition of the metal material, but also related to the geometry and defect properties of the point.5. Solid corrosion product wedge action theoryAccording to this theory, the corrosion product of the metal material precipitates in the rear anode region in the advancing direction of the crack tip, and stress is generated in the crack due to the simple mechanical wedging action of the precipitate. As the deposit increases, the stress increases, and when the stress reaches a critical value, the crack propagates forward. Due to the crack propagation, the chloride ion-containing solution is sucked into the expanded crack, the local anodic corrosion continues to occur, the corrosion product precipitates again, and the stress accumulation again occurs, forcing the crack to expand forward again, and so on. Until all broke. These corrosion products are mostly metal oxy oxides or aqueous metal oxides.According to the results of stress corrosion test on stainless steel, NA Nielsen believes that:a. The wedging effect of the corrosion product forms a tensile stress at the anode notch, which promotes the expansion of the crack;b. The formation of corrosion products promotes the acidity of the medium in the crack, such as: 3Cr+3H2O=Cr2O3+6H+6eSimilarly, iron and nickel are oxidized and hydrolyzed into salts in the presence of Cl-, which promotes the increase of the acidity of the solution in the crack, which is conducive to the expansion of stress corrosion cracking;c. The corrosion product deposited at the crack tip is itself an effective cathode, thus accelerating the anodic dissolution of the crack tip.6. Tunnel-shaped pitting corrosion theoryThis theory suggests that the flow chemistry of these parts is greatly different from the overall chemical composition due to some selective corrosion of the metal surface or due to a special geometry that limits the flow of these parts in the electrolyte. Thereby reducing the potential of these sites, accelerating localized corrosion of the region, forming certain holes in which a so-called occlusion cell is formed, the anode of the activation surface in the occlusion cavity being reflected as:
Figure 5 Schematic diagram of stress adsorption crackingAccording to the stress adsorption theory, we can know:a. For different metal materials, the adsorbed ions are selective. Some ions in some electrolytes are liable to cause stress corrosion cracking of some metal materials. For example, Cl- and HO- are suitable for austenitic stainless steel, N03- or OH- to low carbon steel, etc., which are susceptible to stress corrosion cracking;b. According to the above characteristics, it is possible to replace and dilute the harmful anions with a sufficient concentration of harmless anions. For example, by adding N03- or ions to a solution containing oxygen ions, it can prevent stress corrosion cracking of stainless steel;c. Surface stress adsorption at the crack tip is not only related to the chemical composition of the metal material, but also related to the geometry and defect properties of the point.5. Solid corrosion product wedge action theoryAccording to this theory, the corrosion product of the metal material precipitates in the rear anode region in the advancing direction of the crack tip, and stress is generated in the crack due to the simple mechanical wedging action of the precipitate. As the deposit increases, the stress increases, and when the stress reaches a critical value, the crack propagates forward. Due to the crack propagation, the chloride ion-containing solution is sucked into the expanded crack, the local anodic corrosion continues to occur, the corrosion product precipitates again, and the stress accumulation again occurs, forcing the crack to expand forward again, and so on. Until all broke. These corrosion products are mostly metal oxy oxides or aqueous metal oxides.According to the results of stress corrosion test on stainless steel, NA Nielsen believes that:a. The wedging effect of the corrosion product forms a tensile stress at the anode notch, which promotes the expansion of the crack;b. The formation of corrosion products promotes the acidity of the medium in the crack, such as: 3Cr+3H2O=Cr2O3+6H+6eSimilarly, iron and nickel are oxidized and hydrolyzed into salts in the presence of Cl-, which promotes the increase of the acidity of the solution in the crack, which is conducive to the expansion of stress corrosion cracking;c. The corrosion product deposited at the crack tip is itself an effective cathode, thus accelerating the anodic dissolution of the crack tip.6. Tunnel-shaped pitting corrosion theoryThis theory suggests that the flow chemistry of these parts is greatly different from the overall chemical composition due to some selective corrosion of the metal surface or due to a special geometry that limits the flow of these parts in the electrolyte. Thereby reducing the potential of these sites, accelerating localized corrosion of the region, forming certain holes in which a so-called occlusion cell is formed, the anode of the activation surface in the occlusion cavity being reflected as: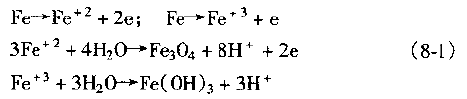 As a result of the anode reflection, the acidity in the cavity is increased, thereby accelerating the pitting corrosion speed. Under the action of the stress, the corrosion hole can be expanded into a crack, and FIG. 8-17 is a schematic diagram of the stress corrosion cracking process caused by the tunnel-shaped pitting corrosion.
As a result of the anode reflection, the acidity in the cavity is increased, thereby accelerating the pitting corrosion speed. Under the action of the stress, the corrosion hole can be expanded into a crack, and FIG. 8-17 is a schematic diagram of the stress corrosion cracking process caused by the tunnel-shaped pitting corrosion.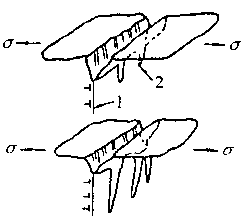 Figure 8-17 Schematic diagram of stress corrosion cracking caused by tunnel-shaped pitting1-slip surface; 2-corrosion tunnel7. Dislocation motion cracking theoryThis theory attempts to explain the phenomenon of stress corrosion cracking from the perspective of metal physics. It is believed that the dislocation motion under stress will cause component segregation and prepare conditions for selective corrosion causing stress corrosion cracking.When the metal causes slippage under stress, the diffusion of alloying elements will accelerate, especially the interstitial atoms such as chlorine, carbon, and hydrogen are easily segregated at the defects. Even some Other elements in the solid solution will accumulate at the defects, forming segregation at the dislocations.The sensitivity of stress corrosion cracking is related to dislocation distribution and stacking fault energy.From the analysis of stainless steel, chrome-nickel alloy and nickel, it is known that the dislocation of layered distribution is more sensitive to stress corrosion cracking than the dislocation of cluster-like distribution. Dislocations with high stacking fault energy are less sensitive to stress corrosion cracking and vice versa. If there are short programs, especially for materials with lower stacking faults. Will increase the sensitivity of stress corrosion cracking.
Figure 8-17 Schematic diagram of stress corrosion cracking caused by tunnel-shaped pitting1-slip surface; 2-corrosion tunnel7. Dislocation motion cracking theoryThis theory attempts to explain the phenomenon of stress corrosion cracking from the perspective of metal physics. It is believed that the dislocation motion under stress will cause component segregation and prepare conditions for selective corrosion causing stress corrosion cracking.When the metal causes slippage under stress, the diffusion of alloying elements will accelerate, especially the interstitial atoms such as chlorine, carbon, and hydrogen are easily segregated at the defects. Even some Other elements in the solid solution will accumulate at the defects, forming segregation at the dislocations.The sensitivity of stress corrosion cracking is related to dislocation distribution and stacking fault energy.From the analysis of stainless steel, chrome-nickel alloy and nickel, it is known that the dislocation of layered distribution is more sensitive to stress corrosion cracking than the dislocation of cluster-like distribution. Dislocations with high stacking fault energy are less sensitive to stress corrosion cracking and vice versa. If there are short programs, especially for materials with lower stacking faults. Will increase the sensitivity of stress corrosion cracking.
Ningbo Kyson Cool Electronic Technology Co., Ltd. , https://www.zjkysoncool.com