Industry experts believe that lithium batteries are "a kind of near-ideal battery" because lithium is the lightest metal on earth, and its ability to carry electrons is superior to lead, zinc, and nickel-cadmium, which are more important substitutes. However, it is undeniable that lithium batteries also have potential safety hazards of overheating and fire. This is also an important reason why lithium air batteries and lithium-sulfur batteries can not be commercially promoted even though they can provide more than 10 times of energy. However, today's Stanford University research team successfully found a way to make lithium batteries safer and is expected to further advance the lithium battery revolution.
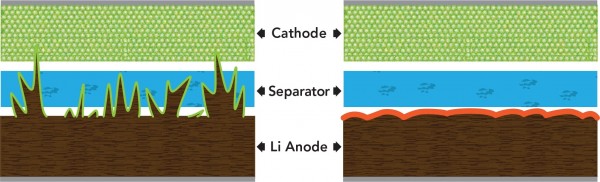
The four main parts of a lithium battery are a positive electrode material, a negative electrode material, a separator, and an electrolyte. When a dendrite or a hand-shaped lithium ion grows inside, it is very easy to cause a short circuit or a random explosion to form an open flame. Once dendrites form inside the cell, they rapidly increase and eventually pierce the positive and negative separators, as shown on the left side of the image above.
To avoid the spontaneous combustion of next-generation batteries, researchers are trying to find new ways to prevent fire hazards. They added two chemical components to the electrolyte inside the battery, one of which is lithium nitrate, which can improve the endurance performance. Another component, lithium polysulfide, can decompose the electrode. During the test, it was possible to control the appearance of harmless pancake-like products inside the cell instead of dendrites. In addition, the two chemical materials also improve the durability of the battery. After 300 battery cycles, the battery capacity still remains over 99%.
Cleaning Scraper,Plaque Scraper,Tooth Scraper,Tongue Cleaner Brush
JIANGSU RARHON TOOLS CO.,LTD , https://www.rarhontools.com