[China Aluminum Industry Net] Aluminum marine corrosion refers to the corrosion behavior of aluminum and aluminum alloys in seawater, marine atmosphere and industrial-ocean atmosphere. Corrosion is damage and deterioration that occurs under the action and influence of the environment. Corrosion causes serious damage to humans. The direct loss caused by corrosion is 1% to 3.5% of the national economy's annual gross production value. In various natural and man-made disasters (fires, earthquakes, fires, etc.), the damage caused by corrosion cannot be ignored. In the first place, and this loss is more subtle, no significant mountain, no significant water, not easy to attract people's attention. In 2015, China's GDP was 676.708 billion yuan. If the direct corrosion loss in the year was estimated at 2.8%, it would be worth 1699.8 billion yuan. This is really not a surprise. One calculation will startle people and make people feel shocked.
Aluminum and most aluminum alloys have a very high resistance to corrosion since very thin layers of dense Al2O3 films, which are only 5 nm thick and have the same colour as aluminum, can be formed extremely rapidly on their surfaces in air. In general, the oxide film is stable in a solution of pH 4.0 to 9.0, and is also stable in concentrated nitric acid (PH=1) and concentrated ammonium hydroxide (PH=13), and the surface of aluminum and industrial pure aluminum is mechanically damaged. Immediately afterwards, a new oxide film will form on the wounded surface and no further corrosion will occur.
The potential of aluminum is determined to a large extent by the insulating properties of the oxide film. Therefore, all factors that can improve the denseness of the oxide film, increase the thickness of the oxide film, and improve the insulation performance of the oxide film improve the corrosion resistance. Conversely, all factors that reduce the effective protection of the oxide film, whether it is mechanical or chemical, will lead to a sharp decline in the corrosion resistance of aluminum and aluminum alloys.
Aluminum alloys are more severe than pure aluminum, but there are few exceptions. The corrosion resistance of aluminum and its alloys in air, acid and tap water increases or decreases in the following order: Al, Al-Mn alloy (3XXX series alloy), Al-Mg alloy (5XXX series alloy), Al-Mg-Si alloy ( 6XXX series alloy), Al-Si alloy (4XXX series alloy), Al-Zn-Mg alloy (7XXX series alloy), Al-Zn-Mg-Cu alloy (7XXX series alloy), Al-Cu-Mg (2XXX series alloy) ), Al-Cu alloy (2XXX series alloy). The descending order in alkaline solution and seawater is: Al-Mg alloy (5XXX series alloy), industrial pure aluminum (1XXX series alloy), Al-Mn alloy (3XXX series alloy), Al-Mg-Si alloy (6XXX series Alloy), Al-Zn-Mg alloy (7XXX series alloy), Al-Si alloy (4XXX series alloy), Al-Zn-Mg-Cu alloy (2XXX series alloy), Al-Cu-Mg alloy (2XXX series alloy) , Al-Cu alloy (2XXX series alloys). However, it should be noted that the above arrangement is rough, and that the order of the alignment is established only when heat treatment is applied to an exfoliation corrosion, intergranular corrosion, or stress corrosion-sensitive alloy and the sensitivity is eliminated.
Alclad refers to rolling a layer of industrial pure or Al-Zn alloy on one or both sides of the alloy sheet. For example, the 2XXX series alloy is coated with a 1A50 alloy and coated on the surface of the 7XXX alloy. It is 7A01 alloy, which is a binary Al-Zn alloy containing 0.9% to 1.3% Zn. The potential of the cladding alloy is more negative than that of the core alloy and can provide electrochemical protection to the core alloy.
Marine atmospheric corrosion of aluminum The main feature of the marine atmosphere is the large amount of salt. Because waves and strong winds bring seawater into the atmosphere, the water evaporates and is blown to the land by the monsoon or typhoon, which increases the salt content in the low-altitude atmosphere and is closely related to the distance from the seashore. The indoor and outdoor salt mist content of the same location is also very different, the latter can be 4 to 6 times larger than the former. In the same area, the suburban areas closer to the sea can differ by about 10 times from the indoor and outdoor areas.
Chlorine and chloride are one of the main impurities in the marine atmosphere. Their content has a great influence on the corrosion of aluminum. For example, 2024 and 7075 alloys are resistant to corrosion in the clean atmosphere, but the atmospheric chlorine content is greater than 1.0%. Their corrosion rate rises sharply, especially when relative humidity RH (relative humidity) is greater than 65%.
The lower the sea, the higher the chloride content of sea fog, the more severe the corrosion of aluminum in the environment, and the chloride accelerates pitting, stress corrosion, intergranular corrosion, and crevice corrosion of aluminum and aluminum alloys. Human sweat contains salt, urea, and lactic acid. They are all media that corrode aluminum and aluminum alloys. Wear gloves when handling, processing, and assembling aluminum and aluminum structures.
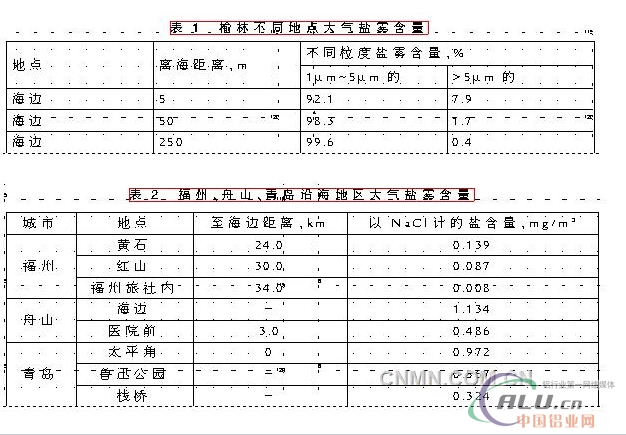
See Table 1 for the content of salt spray with different particle size at different locations in Yulin, Hainan Province, China, and Table 2 for the content of atmospheric salt fog at different locations in Fuzhou, Zhoushan and Qingdao.
Petrochemical Pump,Petrochemical Process Pump,Petrochemical Centrifugal Pump,Petrochemical Industry Pump
Shenyang pump products sales co., LTD , https://www.syipsc.com